-40%
2 Axis Cross Sliding Milling Compound Working Table Bench Drill Vise DIY Tool
$ 24.28
- Description
- Size Guide
Description
Features:
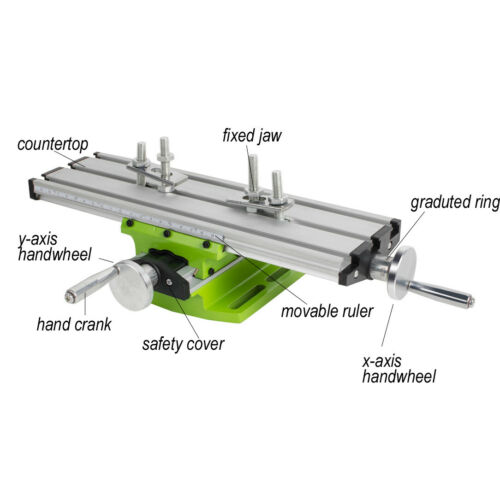
1.The table is suitable for Mini Drill and drill bracket series.
It can also apply to other suitable machines.
2.The work table is composed of several adjustable dovetail slot
aluminum alloy compositions, can guarantee the minimum space and
ensure the accuracy.
3.The working table with three T shaped groove, standard size is
15x8x6mm.
4.Scale is in front of the movable positioning.
5.Zero adjustment handwheel rotates a circle of 1.25mm. A lattice
is equal to 0.05mm.
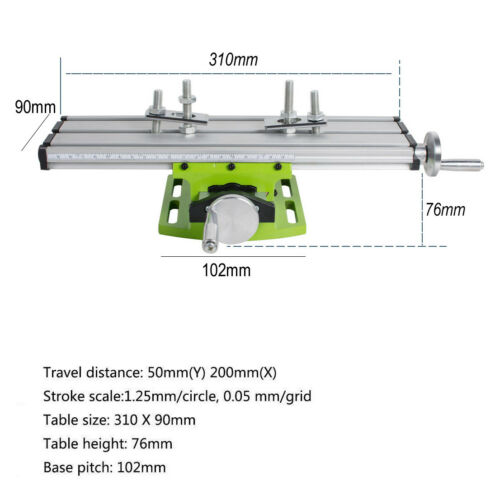
6.The X axis Y axis distance is 200mm, the stroke is 50mm.
7.Working table size is 310x90mm and the height of 76mm, two
working clamps are included.
Specifications:
Material: Aluminium Alloy
Size: 310 * 90mm * 78mm
X-Route: 200mm
Y-Route: 50mm
Distance of grooves: 34mm
Scale travel: 1.25mm (circle), 0.05mm (one cell)
B
ase pitch:102mm
Package Include:
1*Worktable
1* manual
FDA declaration :FDA declaration :Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(CICI-China-86-18328503374)This item has been cleaned and treated according to the manufacturer's instructions.The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892